
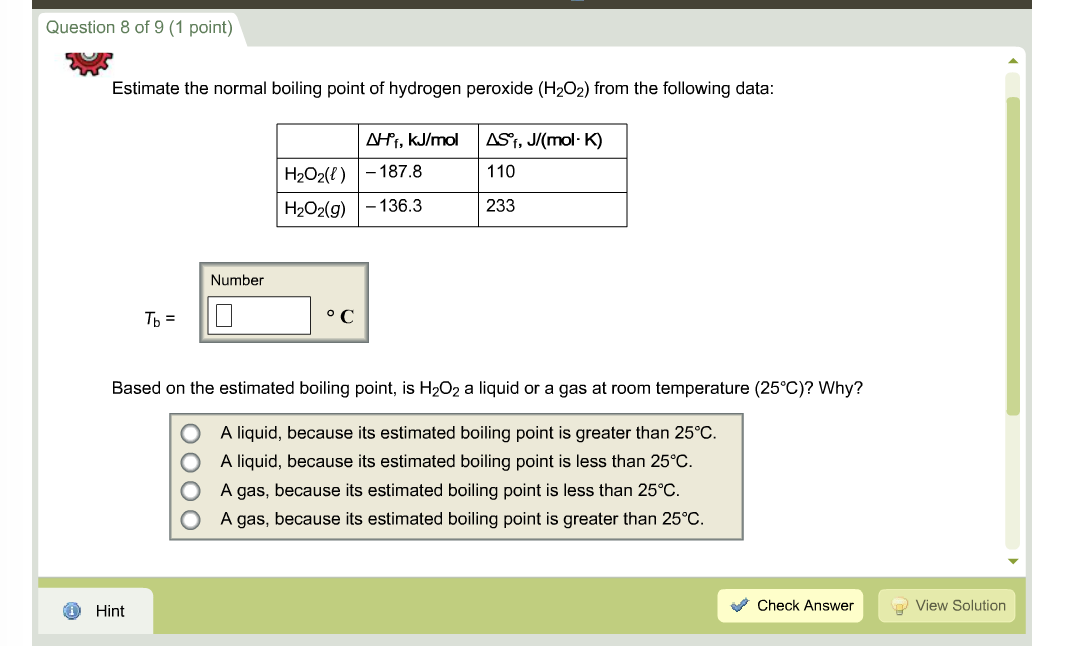
The boiling point of a substance is the temperature at which this phase change (boiling or vaporization) occurs. In general, boiling is a phase change of a substance from the liquid to the gas phase. Temperature given as ☌, ☏, K and °R.Note that, the boiling point associated with the standard atmospheric pressure.
Glycerine - Boiling and Freezing Points - Boiling and freezing points of glycerine aqueous solutions.Gases - Specific Gravities - Specific gravities of air, ammonia, butadiene, carbon dioxide, carbon monoxide and some other common gases.

Gases - Densities - Densities and molecular weights of common gases like acetylene, air, methane, nitrogen, oxygen and others.Fusion and Evaporation Heat of common Materials - Melting points, heat of fusions, boiling points and heat to evaporate common substances - like hydrogen, water, gold and more.Fuels - Boiling Points - Fuels and their boiling points.Temperature - Cavitation of impellers increases with water temperatures. Feeding Pumps in Steam Systems - Suction Lift Head vs.Evaporation from a Water Surface - Evaporation of water from a water surface - like a swimming pool or an open tank - depends on water temperature, air temperature, air humidity and air velocity above the water surface - online calculator.Cryogenic Fluids and Liquefied Gas Properties - Cryogenic properties as density, boiling points and heat of evaporation for fluids like hydrogen, methane, oxygen, nitrogen, fluorine and helium.Condensate Pumping - High temperatures and danger of impeller cavitation is the major challenge for condensate pumping in steam systems.Chemicals - Formulas and Trading Names - Formulas and trading names for some common chemicals.Boiling Liquids - Max Pumping Flow Velocity - Recommended max flow velocity on the delivery (pressure) side when pumping boiling liquids.Boiling Fluids - Max Suction Flow Velocity - Recommended max suction flow velocity when pumping boiling fluids.Ammonia - Vapour Pressure at Gas-Liquid Equilibrium - Figures and table with ammonia saturation pressure at boiling points, SI and Imperial units.Alcohols and Carboxylic Acids - Physical Data - Molweight, melting and boiling point, density, pKa-values, as well as number of carbon and hydrogen atoms in molecules are given for 150 different alcohols and acids.Acetone - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of acetone at temperatures ranging from -95 to 275 ☌ (-138 to 530 ☏) at atmospheric and higher pressure - Imperial and SI Units.
Boiling Points - Boiling points of elements, products and chemical species at varying conditions.Material Properties - Material properties of gases, fluids and solids - densities, specific heats, viscosities and more.Methylene Chloride(CH 2Cl 2, dichloromethane) Alcohol - ethyl (grain, ethanol) C 2H 5OHĪlcohol - methyl (methyl alcohol, wood alcohol, wood naphtha or wood spirits) CH 3OHīrake Fluid Dot 3 (Dry - Wet boiling points) (Wet includes hygroscopic moisture)īrake Fluid Dot 4 (Dry - Wet boiling points)īrake Fluid Dot 5 (Dry - Wet boiling points)īrake Fluid Dot 5.1 (Dry - Wet boiling points)Ĭarbon Tetrachloride (Tetrachloroethane) CCl 4


 0 kommentar(er)
0 kommentar(er)
